 Grant Donovan, MBA President
Grant Donovan, MBA President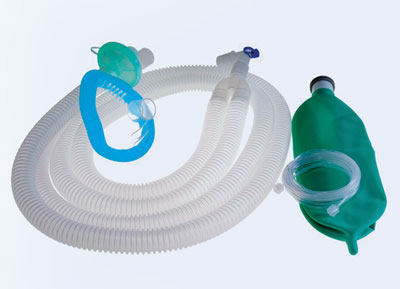
Studying all these adverse effects of PVC, 33 years ago, Marcia Coulson made the decision that she would never manufacture using PVC, keeping her employees and customers safe from the toxic plastic. As such, she founded Eldon James—a specialized manufacturer of PVC-free tubing and connectors.
We are the first to build a company committed to manufacturing PVC-free medical devices. We are answering the call from Healthier Hospitals who’s initiatives are to eliminate PVC from the medical devices
WilMarc has launched customized anesthesia or breathing circuits that include three variants—adult coaxial breathing circuits, adult corrugated breathing circuits, and adult expandable breathing circuits. Designed with higher flexibility, these circuits are meant to prevent the common problems with added dead space (the volume of a breath that does not participate in gas exchange) and the weight of a circle system while allowing the gas to pass freely during anesthesia.
Apart from it, WilMarc has designed anesthesia masks that are free of PVC, DEHP, Bisphenol A (BPA) and are not made from natural rubber latex. “We have completely redesigned the mask to eliminate the cushion that needs to be inflated by the anesthesiologist. It comes with a built-in cushion that helps anesthesiologists to save time,” Coulson elucidates. These masks were extensively tested against the commonly used anesthesia masks. The study revealed that conventional masks hold at least 50,000 phthalate particles, whereas WilMarc’s masks were free of those. In addition to this, the masks are odor-free and come in six color-coded sizes for newborns, infants, toddlers, children, and adults. What’s more? WilMarc is a regular participant in CleanMed, a premier national conference for leaders in health care sustainability. This has helped the company to prove its excellence in implementing climate-smart health care innovations before a large number of audiences.
Honing such capabilities, WilMarc is currently working with a plethora of medical device manufacturers to help them migrate to PVC-free devices. Striding ahead, the company has set the goal to launch Class II medical devices, which will further bolster its presence in the market. “We have decades of experience in designing medical devices combined with our experience in manufacturing PVC-free components. We are listening to hospitals and building PVC-Free medical devices for a safer future,” Coulson concludes.
-
Our core mission is to replace all the PVC-based disposable medical equipment, and for this, we can go to any extent
Honing such capabilities, WilMarc is currently working with a plethora of medical device manufacturers to help them migrate to PVC-free devices. Striding ahead, the company has set the goal to launch Class II medical devices, which will further bolster its presence in the market. “We have decades of experience in designing medical devices combined with our experience in manufacturing PVC-free components. We are listening to hospitals and building PVC-Free medical devices for a safer future,” Coulson concludes.
June 15, 2020





